Preface
Toothpaste constitutes a pivotal element in the domain of oral health and hygiene, serving as a primary barrier against dental caries, periodontal diseases, and other oral maladies. Through the years, the composition of toothpaste has undergone substantial evolution, with two pivotal ingredients often assuming primary importance: fluoride and hydroxyapatite. For an extended period, fluoride, a mineral lauded for its capacity to prevent dental caries, has been the active ingredient in toothpaste. However, a relatively recent addition to the market, hydroxyapatite, has emerged as a new contender, offering a comparable level of protection with additional benefits.This article undertakes a comprehensive examination of the distinctions between fluoride and hydroxyapatite in toothpaste.The subsequent sections delve into the functions and actions of these compounds, their underlying mechanisms, their effectiveness, potential risks, and associated costs. The objective of this article is to elucidate the properties and functions of these compounds, providing the reader with the necessary information to make an informed decision when selecting a toothpaste.This exploration offers a comprehensive overview of the scientific principles underlying daily dental care products and practices, catering to a diverse audience, including dental professionals, health-conscious consumers, and casual readers.
What is Fluoride?
The utilization of fluoride in dental care has a long history. The initial identification of fluoride's potential benefits in dentistry occurred in the early 20th century, when scientists observed a correlation between regions with high fluoride water and reduced tooth decay rates. This discovery led to the implementation of water fluoridation, a public health initiative that began in the United States during the 1920s.
The advent of fluoride-containing toothpastes in the 1950s marked a significant milestone in the evolution of dental care. The World Health Organization and other health bodies now recognize fluoride as a crucial component of oral health.
How does Fluoride Toothpaste Work?
Fluoride toothpaste has been demonstrated to facilitate the remineralization of tooth enamel, which constitutes the outermost protective layer of teeth. The process of remineralization is initiated when fluoride ions present in toothpaste interact with saliva within the oral cavity. This reaction results in the formation of a transient fluoride layer on the teeth, thereby providing a protective barrier to the enamel.
Subsequently, fluoride undergoes a process of ion exchange with the tooth enamel, thereby replenishing the minerals that have been depleted and reducing the enamel's vulnerability to acid solutions produced by plaque bacteria and sugars present in the oral cavity. This process contributes to the prevention of dental caries and cavities.
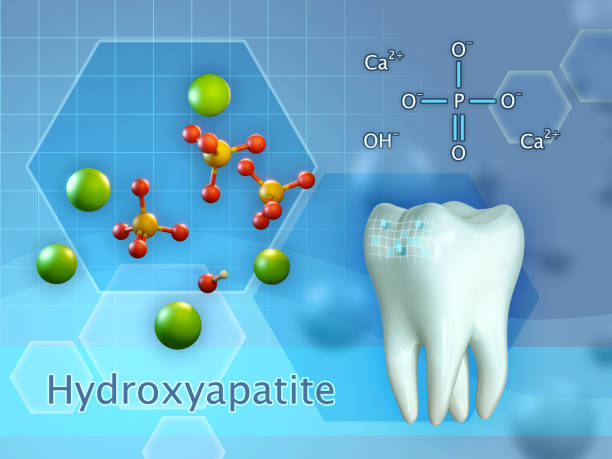
What is Hydroxyapatite (HAp)?
Hydroxyapatite, abbreviated as HAp, is a bio mineral that occurs naturally with tooth enamel and dentine. It is also present in bones and accounts for approximately 70% of the bone matrix. HA is a calcium and phosphate mineral that is characterized by an oxygen matrix.
Of particular relevance in the domain of dental health is the bio-compatibility of hydroxyapatite with the human body, as well as its capacity to fortify and repair tooth enamel.
How Does Hydroxyapatite Toothpaste Work?
As with fluoride, hydroxyapatite toothpaste has been demonstrated to strengthen and rebuild tooth enamel, albeit through a distinct mechanism. The nanoscale particles of the mineral present in hydroxyapatite toothpaste have the capacity to adhere to the tooth enamel.
Upon application, these particles are deposited onto the surface of the teeth, thereby masking any surface irregularities present on the enamel. Furthermore, it has been demonstrated to rebuild small amounts of tooth structure that have been lost due to decay, protect the tooth from future decay, and make teeth appear whiter by creating a shiny surface.
How to use Hydroxyapatite Toothpaste?
Choose the right product: First, choose the right nano-hydroxyapatite toothpaste with a good quality and from a good company. Search for products that have nano-hydroxyapatite stated clearly on the pack and look for other components that may be suitable for your dental problems, such as, for example, for sensitive teeth or natural whitening substances.
Brush regularly: Apply nano-hydroxyapatite toothpaste at least twice a day. Place a small drop of the toothpaste on the head of the soft bristle toothbrush.
Use proper brushing technique: Circular motions should be used while brushing with applying pressure to the tooth’s surfaces. The nano-hydroxyapatite needs at least two minutes to bond with the tooth enamel during each brushing session.
Don't rinse immediately: Spit out the toothpaste after brushing but do not rinse your mouth with water immediately after brushing. If you are able to leave a thin layer of toothpaste on your teeth, it will continue to remineralize for a longer period. If the feeling of not rinsing is too uncomfortable you can rinse with water or use a mouthwash that reacts with remineralizing toothpastes.
Consistency is key: This is true for any dental hygiene product and therefore it is advisable to use it regularly. The advantages of using nano-hydroxyapatite are realized after a long period of usage.
Complement with good dental habits: Use nano-hydroxyapatite toothpaste, floss daily, use an antibacterial mouthwash, and consume low acidic and sugary products for the best dental care.
Regular dental checkups: Keep seeing your dentist for cleaning and check up as you had been doing before. Consult your dentist about the use of nano-hydroxyapatite toothpaste to see if it is right for your dental hygiene routine.
Fluoride vs. Hydroxyapatite: Which is Better?
| Technical aspects |
Fluoride |
Hydroxyapatite |
| Effectiveness |
Fluoride has been extensively studied and proven to be effective in preventing tooth decay and cavities. It works by promoting the remineralization of tooth enamel, making teeth more resistant to acid attacks. |
Hydroxyapatite has also been shown to be effective in preventing tooth decay. It works by binding directly to tooth enamel, filling small spaces and crevices. Some studies suggest it may be as effective as fluoride, but these results are fewer and often small-scale. |
| Safety |
Generally safe for most people when used appropriately. However, high concentrations or accidental ingestion can lead to health problems, including dental fluorosis in children and potential bone and neurological problems in extreme cases. Some people also have ethical objections to the use of fluoride. |
Hydroxyapatite is considered safe and biocompatible, with no known health risks. It is not associated with any toxicity or overexposure concerns because it is a natural component of human teeth and bones. |
| Accessibility |
Il dentifricio al fluoro è ampiamente disponibile e generalmente conveniente, disponibile in una vasta gamma di marche e fasce di prezzo. |
Il dentifricio all'idrossiapatite tende ad essere più costoso del dentifricio al fluoro. Si trova generalmente nei prodotti premium o speciali per l'igiene orale. |
| Availability |
Fluoride toothpaste is widely available throughout the world, in supermarkets, pharmacies and online stores. |
Hydroxyapatite toothpaste, while growing in popularity, is not as readily available as fluoride toothpaste. It is more likely to be found in health food stores, specialty dental stores, or online. |
How Does Hydroxyapatite Compare to Fluoride in Terms of Effectiveness?
Fluoride and hydroxyapatite have been demonstrated to be effective in preventing tooth decay. However, a greater volume of research has been conducted on fluoride, and it has been utilized in dentistry for a more protracted period.
Fluoride exerts its action by accelerating the process of remineralization of tooth enamel, thereby fortifying teeth and reducing their vulnerability to decay. In contrast, hydroxyapatite functions by adhering to tooth enamel, thereby filling the tiny pores and cracks.
A small number of studies have suggested that hydroxyapatite toothpaste can be as effective as a fluoride toothpaste in reducing the risk of caries. However, the number of studies focusing on hydroxyapatite toothpaste is limited, and the existing studies are less extensive than those examining fluoride toothpaste.
Benefits of Hydroxyapatite over Fluoride Toothpastes
Nonetheless, the utilization of hydroxyapatite toothpastes does offer certain advantages, and the efficacy of fluoride has been well-documented. First, hydroxyapatite has not been reported to cause any adverse effects, which may be a preferable option for individuals concerned about the potential side effects of fluoride.
Secondly, the potential for more natural
teeth whitening is suggested by the reduction in enamel roughness and enhancement of enamel reflectance capacity. This is a clear advantage in terms of the appearance of many devices for many users. Finally, the potential reduced toxicity of hydroxyapatite toothpaste is notable, as it is a naturally occurring substance in human teeth and bones, suggesting a more gentle mode of action.
Is fluorapatite stronger than hydroxyapatite?
In fact, fluorapatite exhibits slightly higher strength and resistance to acid than hydroxyapatite. This enhanced property can be attributed to the ability of fluoride ions to displace hydroxide ions within the structure of hydroxyapatite, resulting in the formation of fluorapatite. This transition renders fluorapatite more stable and less soluble compared to its precursor. This principle underlies the efficacy of fluoride in dental products such as toothpaste, which promote the formation of a fluorapatite layer on the teeth's surface. This increased resistance to acid attacks, in turn, reduces the likelihood of dental caries.
Should You Use Fluoride or Non-fluoride Toothpaste?
Fluoridated and non-fluoridated toothpaste are well established and the decision which one to use should be made based on some factors. It is recommended that people who are most prone to cavities, including those who have cavities, should use fluoridated toothpaste. However, for those who have ethical or health problems with fluoride, there is hydroxyapatite toothpaste or any other non-fluoride type.
What are the Benefits of Using Fluoride Toothpaste?
A substantial body of research has demonstrated a correlation between the use of fluoride toothpaste and a reduced incidence of dental caries. The presence of fluoride in toothpaste has been demonstrated to enhance the process of remineralization, thereby increasing the hardness of tooth enamel and reducing its susceptibility to acid attack. Furthermore, it has been demonstrated to be efficacious in reversing early-stage cavities.
It is particularly recommended for individuals with a history of dental cavities, poor oral hygiene practices, or a diet high in sugary foods and beverages. It is also recommended for children, whose teeth are still growing and more susceptible to cavities.
The Process of Remineralization and Demineralization
Demineralization and remineralization are ongoing processes within tooth enamel. The consumption of foods results in the reaction of sugars with bacteria present in the oral cavity, leading to the formation of acids. This process, known as demineralization, involves the dissolution of mineral components from tooth enamel, leading to the formation of cavities and subsequent tooth decay.
However, this process is inherently reversible through a process known as remineralization. Saliva contains minerals such as calcium and phosphate, which are deposited on the outer surface of the enamel. The process of remineralization is facilitated by fluoride, which can be obtained from various sources such as toothpaste, water, or other sources. Fluoride forms a more acid-resistant mineral known as fluorapatite on the surface of the tooth, thereby contributing to the restoration of enamel integrity.
Conclusion
Fluoride:
Public Water Fluoridation and Its Community-Based Effectiveness in Reducing Dental Disease in Children – This article by Armfield JM, published in "Public Health Reports" in 2010, elucidates the effectiveness of fluoridation of water in mitigating tooth decay in children. In light of these findings, it is evident that fluoridation of water plays a significant role in the prevention of tooth decay.
The following article presents a summary of updated clinical recommendations and a supporting systematic review of topical fluoride for cavity prevention: "The Journal of the American Dental Association," 2013. The systematic review by Weyant RJ et al. demonstrated the professional application of fluoride gel to be beneficial in reducing the occurrence of dental caries in high-risk patients.
Hydroxyapatite:
Remineralization Effects of Xylitol on Demineralized Enamel – This experiment, conducted by Mei ML et al in the "Journal of Electron Microscopy" in 2013, demonstrated that microcrystalline hydroxyapatite can facilitate the remineralization of enamel and may be a viable treatment for dental caries.
Hydroxyapatite and its Use as a Bone Graft Material – In this review, the author LeGeros RZ provides information on the use of hydroxyapatite in bone grafting and its possible application in dentistry. The article was published in the "Dental Clinics of North America" in 1991. The review indicates that hydroxyapatite possesses characteristics that can be applied in dental restorative processes.
A study by Pradeep AR et al., published in the Journal of Periodontology in 2010, evaluated the clinical effectiveness of sodium calcium phosphosilicate-containing dentifrice (Novamin) and potassium nitrate-containing dentifrice in treating dentinal hypersensitivity. The study concluded that a hydroxyapatite-containing dentifrice was effective in managing this condition.

























Whatsapp: +8615920313473
cndoublewhite
Doublewhite
doublewhitecn
cndoublewhite
cndoublewhite